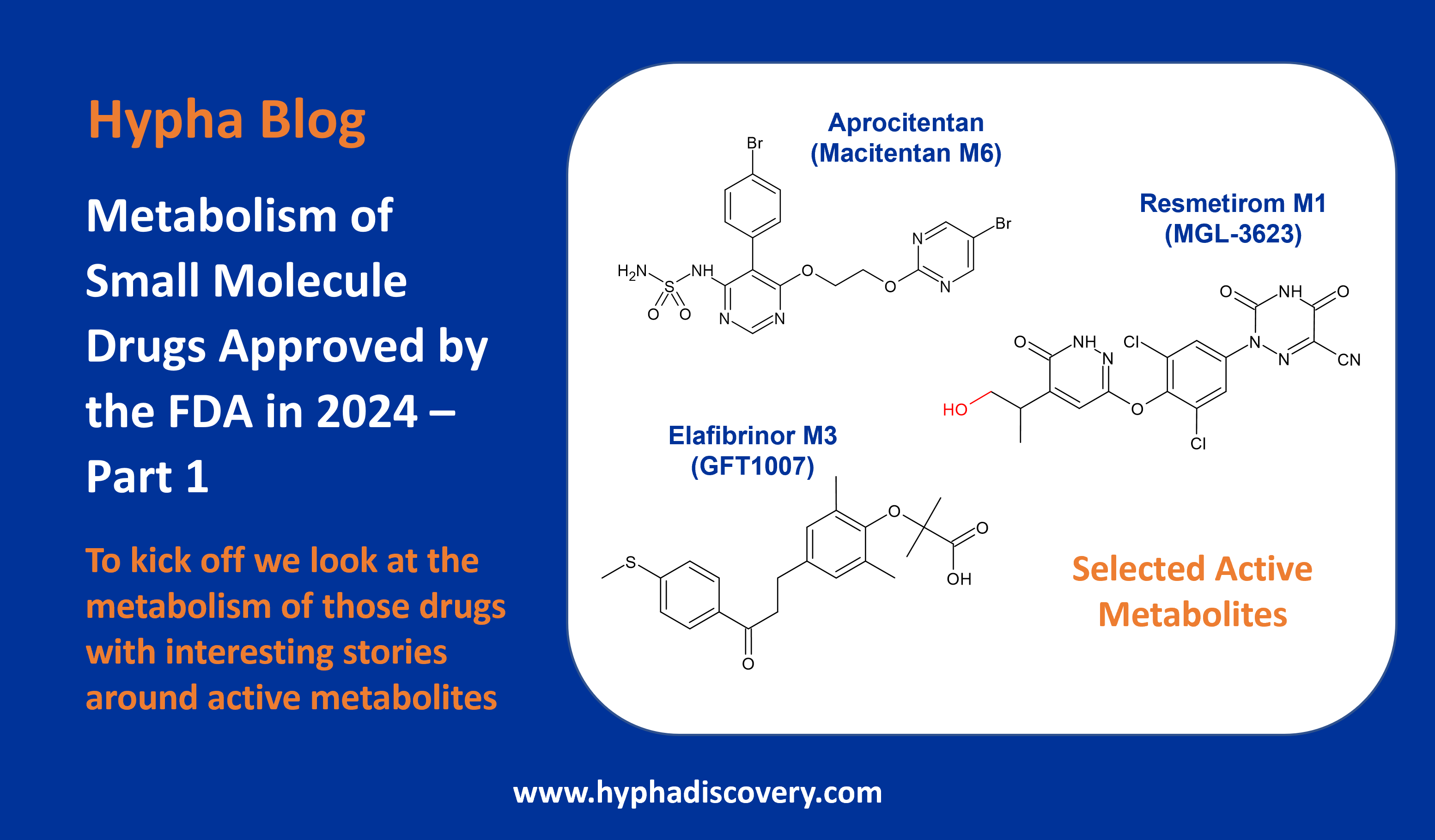
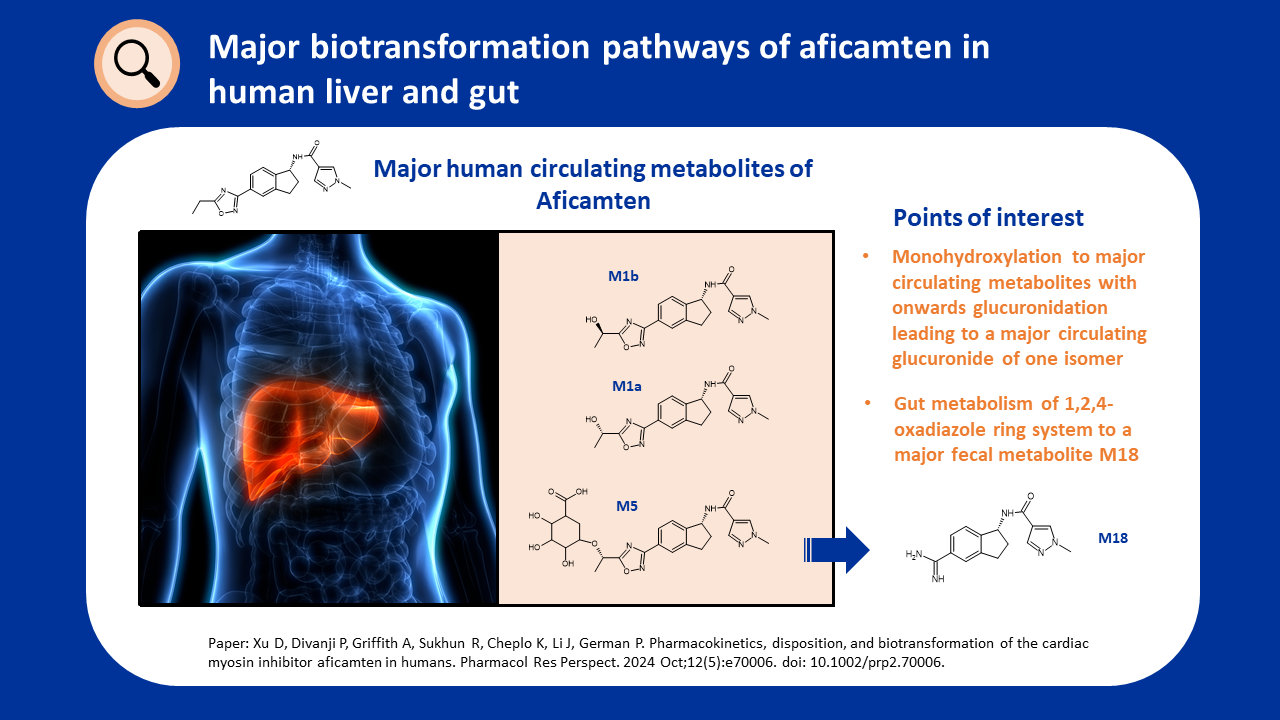
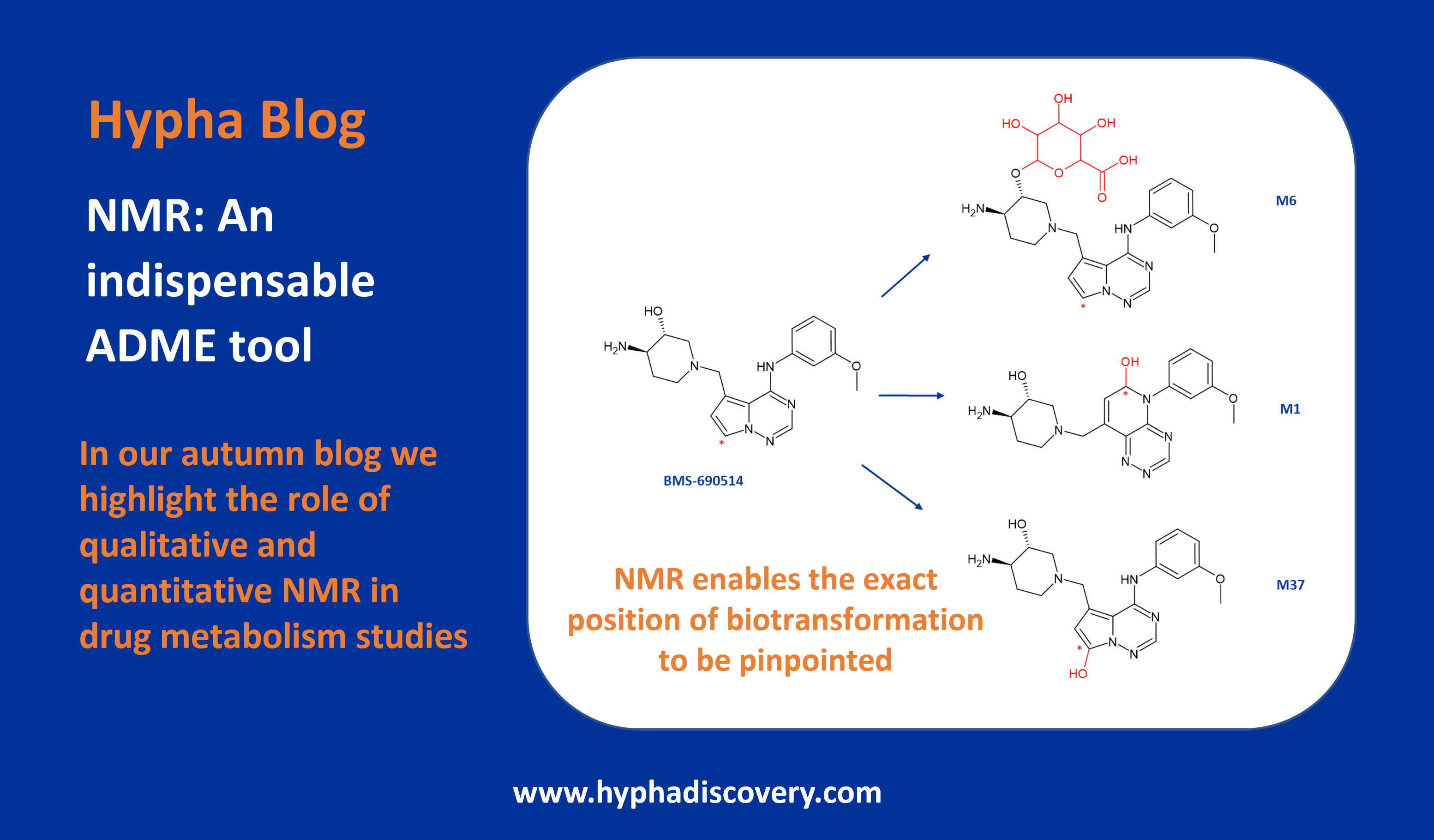
CYP3A-Mediated Metabolism of Suzetrigine
By Julia Shanu-Wilson
It’s been more than 20 years since a pain drug with a new mechanism has been given the go-ahead. As a result there’s been much excitement around the recent FDA approval of Vertex’s suzetrigine as a first-in-class non-opioid treatment for management of acute pain.
Suzetrigine (marketed as Journavx) selectively blocks the NaV1.8 voltage-gated sodium channel, thereby preventing nerve pulses from the peripheral nervous system from reaching the brain and being interpreted as pain. The drug is touted as a possible game changer in the battle against addiction to opoid-based painkillers, although trials are still ongoing exploring suzetrigine’s effectiveness with chronic pain.
An active metabolite
Interestingly the prescribing information for suzetrigine (VX-548) reveals the presence of a major active metabolite, M6-SUZ. The metabolite is a less potent inhibitor of NaV1.8 than the parent drug by 3.7-fold, based on an in vitro electrophysiology assay in human dorsal root ganglion neurons [1]. The primary pathway involved in formation of M6-SUZ is via CYP3A.
Although the structure of the human active metabolite is not described, in vitro studies with rat liver microsomes reveal formation of two main metabolites, an O-desmethyl metabolite (M1) and an N-oxide (M2) [2].
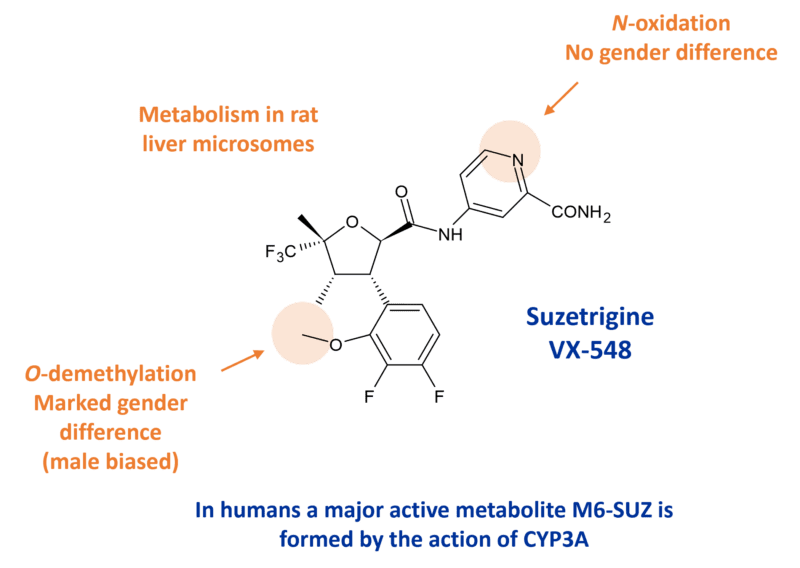
Differential clearance in male vs female rats
In the same paper, inhibition experiments in human liver microsomes indicated that CYP3A was the major enzyme involved in metabolism of VX-548. The study in rats revealed a significant gender difference in metabolism, with male rats clearing the drug much faster than females. In vitro liver microsome studies confirmed a much slower metabolism in female rats with the gender difference attributed to a lowered ability to convert the drug into the desmethyl metabolite. Enzyme studies with rat CYPs revealed that the formation of the desmethyl metabolite was mainly catalysed by CYP3A2 and CYP2C11, CYPs largely expressed in male rats. Rat CYP3A2 is an orthologue of human CYP3A4 and is known to undertake oxidative demethylation.
Gender dependent CYP3A4 metabolism in humans
Gender dependent expression of CYPs in rats is well known, leading to gender-dependent metabolism of drugs [3, and references therein]. Gender differences also exist in humans, with several studies showing that women have higher hepatic and intestinal CYP3A4 activity compared to men. Although a study by Parkinson et al. showed there were no statistically significant differences in CYP3A4 activity in human liver microsomes, there was a significant gender difference in CYP3A4 activity in cryopreserved human hepatocytes (females = twice males) [4].
The potential differences in clearance of the suzetrigine in rats highlight the potential for drugs metabolised by CYP3A4 to be differentially cleared in humans based on gender differences. However, based on published studies for most CYP3A substrates, gender does not appear to influence clearance in humans; except for specific substrates where significant sex-related differences can be seen, with women generally having higher clearance than men [5].
Sex differences in metabolic enzyme activity is only one possible element responsible for variability in pharmacokinetics (PK). Although the impact of gender on PK and PD is becoming more appreciated as more examples of sex-specific differences are reported, much more research is needed to understand disparities, both for existing drugs and those just coming onto the market [6].
References
[1] HIGHLIGHTS OF PRESCRIBING INFORMATION for JOURNAVX (suzetrigine). https://www.accessdata.fda.gov/drugsatfda_docs/label/2025/219209s000lbl.pdf
[2] Yu G, Zhou X. Gender difference in the pharmacokinetics and metabolism of VX-548 in rats. Biopharm Drug Dispos. 2024;45(2):107-114. https://doi.org/10.1002/bdd.2387
[3] Gerges SH, El-Kadi AOS. Sexual dimorphism in the expression of cytochrome P450 enzymes in rat heart, liver, kidney, lung, brain, and small intestine. Drug Metab Dispos. 2023;51(1):81-94. https://doi.org/10.1124/dmd.122.000915
[4] Parkinson A, Mudra DR, Johnson C, Dwyer A, Carroll KM. The effects of gender, age, ethnicity, and liver cirrhosis on cytochrome P450 enzyme activity in human liver microsomes and inducibility in cultured human hepatocytes. Toxicol Appl Pharmacol. 2004;199(3):193-209. https://doi.org/10.1016/j.taap.2004.01.010
[4] Cotreau MM, von Moltke LL, Greenblatt DJ. The influence of age and sex on the clearance of cytochrome P450 3A substrates. Clin Pharmacokinet. 2005;44(1):33-60. https://doi.org/10.2165/00003088-200544010-00002
[6] Oi Yan Chan J, Moullet M, Williamson B, Arends RH, Pilla Reddy V. Harnessing clinical trial and real-world data towards an understanding of sex effects on drug pharmacokinetics, pharmacodynamics and efficacy. Front Pharmacol. 2022;13:874606. https://doi.org/10.3389/fphar.2022.874606