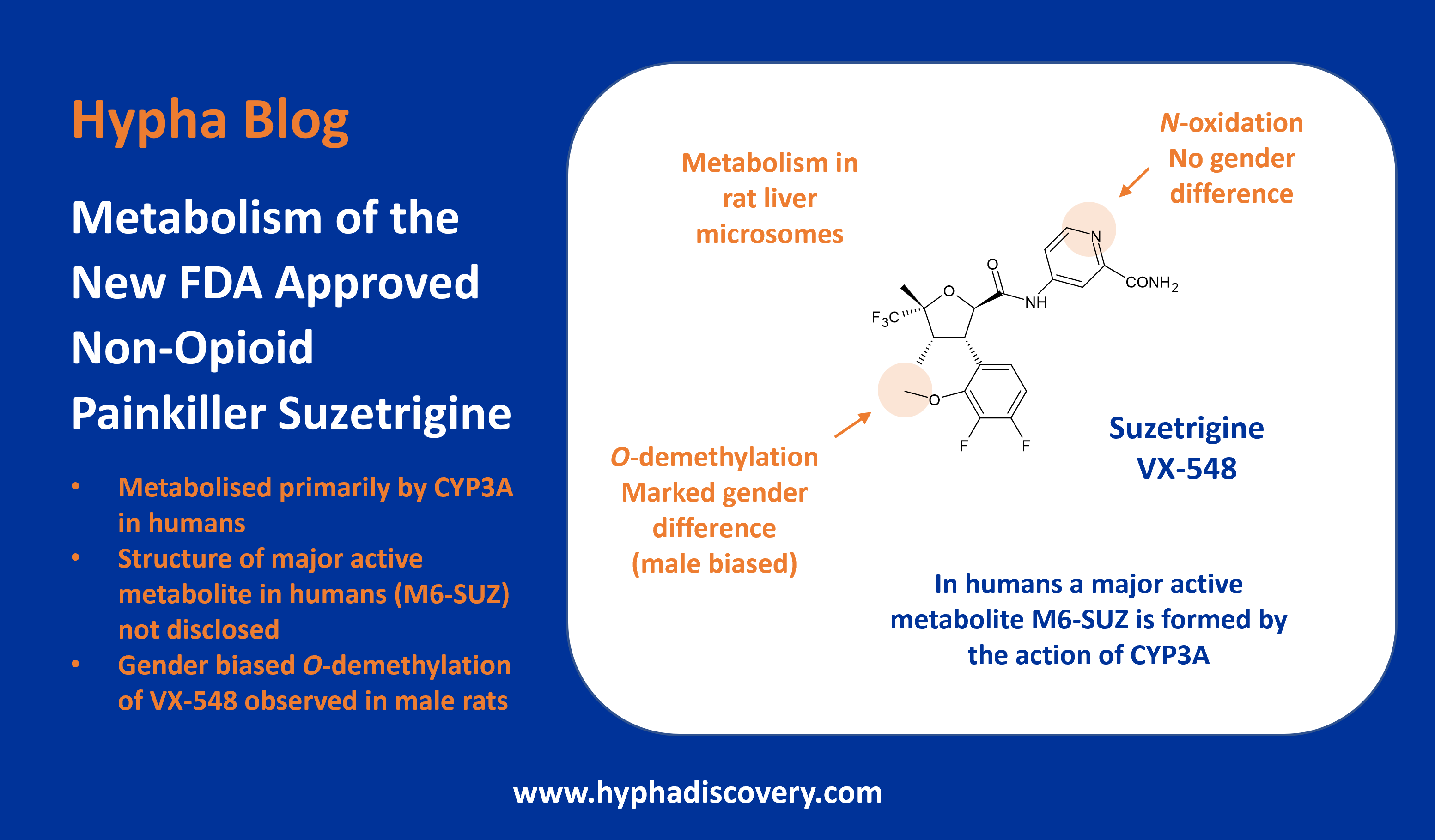
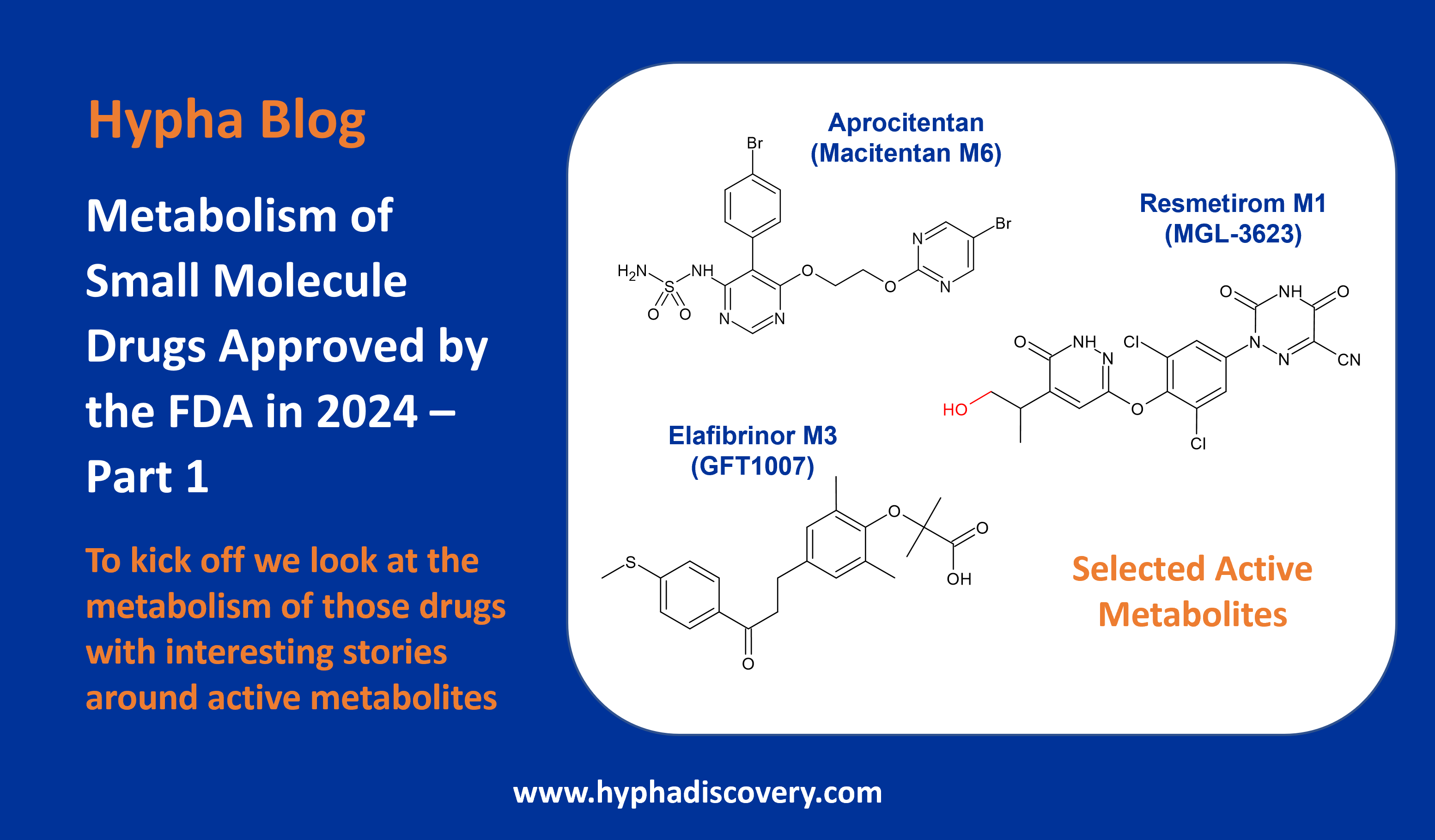
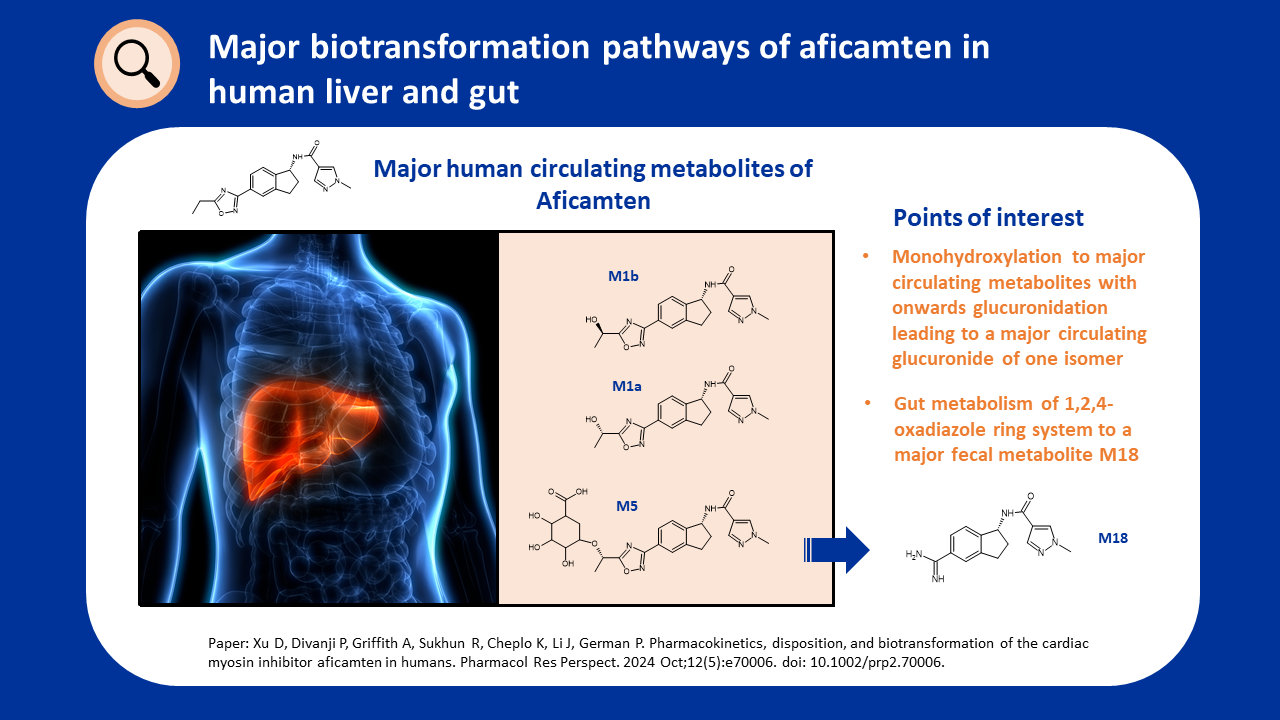
Metabolism of PROTACs
Common biotransformation pathways
By Julia Shanu-Wilson
Heterobifunctional protein degraders such as proteolysis targeting chimeras (PROTACs) comprise a growing group of compounds that exist in the bRo5 (beyond Lipinski’s rule of five) physicochemical space. A speaker at a recent meeting focussing on the DMPK challenges of these types of molecules highlighted that 20 PROTACs are in or entering the clinic as of May 2024!
As expected, metabolism of the linker unit is observed, and, according to another speaker at the same meeting, can result in polar metabolites that are cleared so slowly that they are present at higher levels than the parent drug.
Due to the size of these molecules and the accommodating active site of CYP3A4/5, this enzyme is expected to be heavily involved in metabolism. Involvement of cytochrome P450s (CYPs) is supported by a paper by Volak et al., in which a survey conducted with 18 IQ DMPK/Safety Working Group members revealed that the vast majority (17 out of 18) observed metabolism via this route [1]. Goracci et al. showed that CYP3A4 could undertake cleavage and dehydrogenation reactions of the linker unit in 6 PROTACs studied [2].
Oxidation is not the only route of metabolism observed according to the same survey. Involvement of UGTs is also a significant route to formation of glucuronide conjugates of these drugs. Glucuronidation as a relevant metabolic route (as well as oxidation and hydrolysis) of PROTACs is supported by the types of metabolites Hypha has been requested to make for these type of molecules.
The survey in the Volak paper also revealed the involvement of amidase, esterases, aldehyde oxidase, glutathione-S-transferase and hydrolytic metabolic pathways. In fact enzymatic clearance was seen in more than 70% of responses.

The involvement of aldehyde oxidase was explored by Goracci et al., since moieties vulnerable to this enzyme such as amide groups and heteroaromatic rings are present in PROTACs. Experiments showed that the 5-phenyl-thiazole moiety in the VHL ligand [3] is hydroxylated by aldehyde oxidase [2].
Effect on pharmacology
In the Volak paper, attention was also paid to the relevance of pharmacological activity of metabolites. Of those that measured metabolites, 28% observed effects on pharmacology, largely due to separation of the ligase binding motif from the target binding motif. Interestingly one respondent observed a unique effect arising from interference of the metabolite in the pharmacology. Hence a recommendation in the paper is that metabolite identification should be done prior to in vivo pharmacology studies due to the potential for metabolites to interfere in primary pharmacology.
 In a paper by Hayhow et al., rigorous efforts identified “metabolite 8” of AZ ‘6421 as a significant “interference” in the pharmacology of the degrader [4]. The metabolite lacks the VHL binding moiety due to linker cleavage resulting in formation of a carboxylic acid formed from oxidation adjacent to the ether in the linker. Metabolite 8 is more polar than the parent PROTAC, present in substantial free quantities in vivo, whilst also having equal binding potency and a longer half-life.
In a paper by Hayhow et al., rigorous efforts identified “metabolite 8” of AZ ‘6421 as a significant “interference” in the pharmacology of the degrader [4]. The metabolite lacks the VHL binding moiety due to linker cleavage resulting in formation of a carboxylic acid formed from oxidation adjacent to the ether in the linker. Metabolite 8 is more polar than the parent PROTAC, present in substantial free quantities in vivo, whilst also having equal binding potency and a longer half-life.
The authors also point out the risk of metabolites exhibiting discrete pharmacology different to that of the parent. In fact experiments indicated that metabolite 8 is a lesser degrader of ERα and, unlike the parent, acts as an agonist in Isikawa endometrial cells in vitro.
 The observation that metabolites form adjacent to heteroatoms in the linker, as seen for AZ’6421, was highlighted previously in the Goracci paper [2]. The authors also reported the involvement of aldehyde oxidase in PROTAC metabolism, specifically involving hydroxylation of the a 4-methyl-5-phenyl-thiazole moiety of VHL ligands. Hydroxylation of this moiety is also evident in metabolism of the PROTAC MZ1.
The observation that metabolites form adjacent to heteroatoms in the linker, as seen for AZ’6421, was highlighted previously in the Goracci paper [2]. The authors also reported the involvement of aldehyde oxidase in PROTAC metabolism, specifically involving hydroxylation of the a 4-methyl-5-phenyl-thiazole moiety of VHL ligands. Hydroxylation of this moiety is also evident in metabolism of the PROTAC MZ1.
It will be interesting to see how the metabolism landscape of these degraders unfolds as more of these drugs advance through clinical studies.
References
[1] PK and ADME Characterization of Targeted Protein Degraders. Laurie P. Volak, Heide Marika Duevel, Sara Humphreys, David Nettleton, Colin Phipps, Andy Pike, Caroline Rynn, Paul Scott-Stevens, Donglu Zhang and Michael Zientek. Drug Metabolism and Disposition, 2023, 51 (7) 792-803; DOI: https://doi.org/10.1124/dmd.122.001154
[2] Understanding the Metabolism of Proteolysis Targeting Chimeras (PROTACs): The Next Step toward Pharmaceutical Applications. Laura Goracci, Jenny Desantis, Aurora Valeri, Beatrice Castellani, Michela Eleuteri, and Gabriele Cruciani. Journal of Medicinal Chemistry, 2020, 63 (20), 11615-11638. https://doi.org/10.1021/acs.jmedchem.0c00793
[3] Discovery of small molecule ligands for the von Hippel-Lindau (VHL) E3 ligase and their use as inhibitors and PROTAC degraders. Diehl CJ, Ciulli A. Chem Soc Rev. 2022 Oct 3;51(19):8216-8257. doi: 10.1039/d2cs00387b.
[4] Metabolism-driven in vitro/in vivo disconnect of an oral ERɑ VHL-PROTAC. Hayhow, T.G., Williamson, B., Lawson, M. et al. Commun Biol 7, 563 (2024). https://doi.org/10.1038/s42003-024-06238-x