Acyl Glucuronides of Carboxylic acid-containing Drugs
The FDA’s Safety Testing of Drug Metabolites Guidance for Industry states that phase 2 conjugates are generally pharmacologically inactive, however where a potentially toxic conjugate, such as an acyl glucuronide is formed, additional safety assessments may be needed. Idiosyncratic drug toxicity of carboxylic acid-containing drugs can be caused by the formation of reactive acyl glucuronides,1 which have the ability to directly acylate proteins and undergo intramolecular rearrangement producing reactive aldehydes leading to protein glycation.
Further, there is evidence to suggest that on-target pharmacological studies of acyl glucuronides of drugs are also warranted.2 This is particularly relevant where acyl glucuronidation constitutes the primary clearance mechanism, or where the pharmacological target is in the extracellular matrix and does not require penetration by the acyl glucuronide conjugate.
Also relevant is where multiple acyl glucuronides may be formed from a parent drug through the parallel action of oxidation and glucuronidation. A paper by Dennis Smith and authors on safety assessment of acyl glucuronides, highlight that humans, who readily oxidise acidic drugs, may potentially have abundant acyl glucuronides of oxidative metabolites of the drug in circulation and excreta. In contrast, in rat the oxidative metabolites, and in dog the acyl glucuronide of the parent, may be the major forms detected.
Glucuronides can also be responsible for clinically relevant DDIs
Glucuronides can also be responsible for clinically relevant DDIs, such as those attributed to the acyl glucuronides of clopidogrel3 and gemfibrozil4, which selectively inhibit CYP2C8. As humans readily oxidise acidic drugs, there is also a potential complication arising from the presence of acyl glucuronides of oxidative metabolites of the drug, and which may later alter conjugate reactivity if oxidation occurs on a moiety nearby.4 Further issues can arise due to β-glucuronidase-mediated hydrolysis of the parent drug, the propensity for which differs due to marked species differences in expression of β-glucuronidases.5
Acyl glucuronides in idiosyncratic drug toxicity
"If the conjugate forms a potentially toxic compound such as acyl glucuronide, additional safety assessment may be needed." (FDA’s MIST guidance March 2020).
Acyl glucuronides in DDIs
Several classes of glucuronide conjugates, which include acyl glucuronides, O-glucuronides, N-glucuronides, and carbamoyl glucuronides, have been shown to be substrates or time-dependent inhibitors of CYP2C8 (Ma et al., 2017)
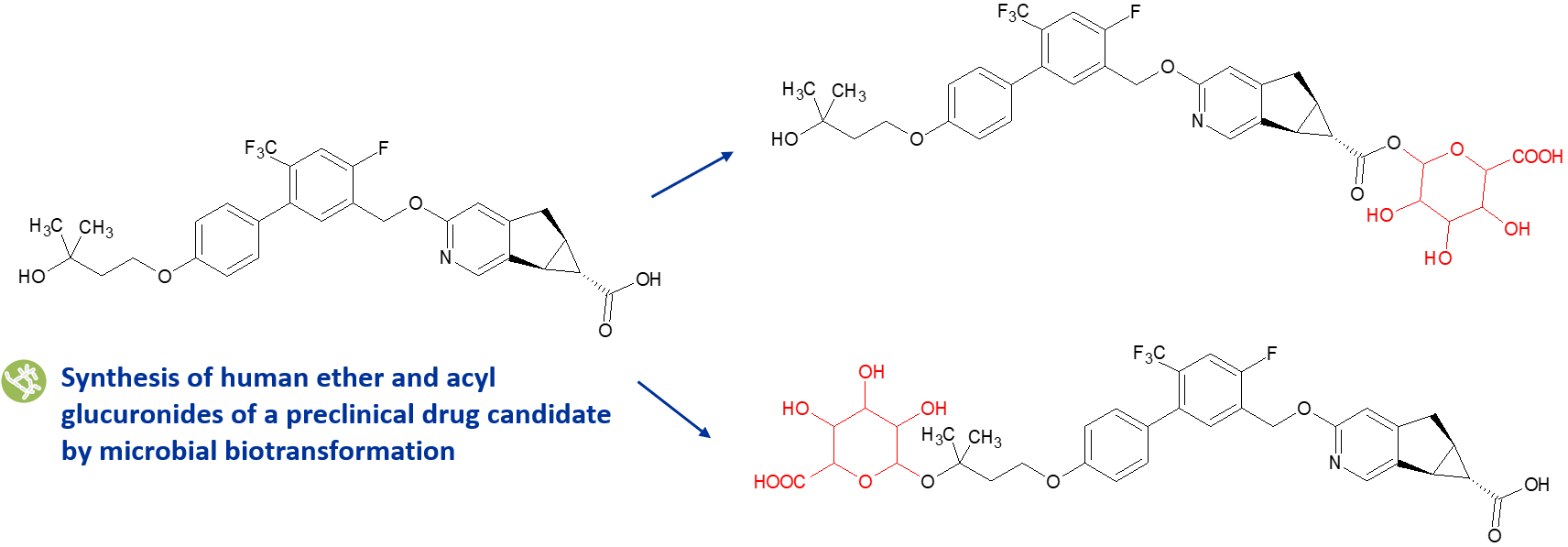
We use both late-stage chemical synthesis and biotransformation methods to make acyl glucuronides for clients. We have designed our methods to minimise instability resulting from the formation of acyl migration isomers.
In the client project illustrated above, quantities of both the acyl and ether glucuronides were needed to study pathways responsible for the drug candidate’s clearance. A number of Hypha’s microbes were able to produce both glucuronides in good yields. Due to chemical intractability of the ether glucuronide, a streptomycete strain was scaled up to provide the metabolite, which was identical to that formed by incubation of the parent compound with recombinant human UGT1A4.6
We have several proprietary late-stage chemical synthesis methods for making acyl glucuronides. In a recent project for a client, screening of a drug in a number of conditions pinpointed a synthetic method that enable scale-up to provide 100s of mgs of the acyl glucuronide, as well as tens of mgs of the deuterated metabolite. Cryoprobe NMR was used to confirm the structure and certificates of analysis were provided for the purified material.
References
- Lassila et al., 2015. Chem Res Toxicol 28(2):2292-23032.
- Ryder et al., 2018. J Med Chem 61(16):7273-7288
- Tornio et al., 2014. Clin Pharmacol Ther 96(4):498-507
- Ogilivie et al., 2006. Drug Metab Dispos 34(1):191-197
- Smith et al., 2018. Drug Metab Dispos 46(6):980-912
- Salter et al., 2018. Xenobiotica 21:1-10
Related Resources
Formation and scale-up of human metabolites formed through mixed metabolic pathways is possible using Hypha’s microbial biocatalysis system. In vivo human metabolism of Incyte’s IND epacadostat (EPA) forms 3 major circulating metabolites, from both primary and secondary pathways. Glucuronidation of EPA forms M9, the dominant metabolic pathway, in conjunction with formation of an amidine M11 and an N-dealkylated metabolite, M12.
Hypha’s microbial biocatalysis process is effective at generating metabolites at up to gram scale. Through Hypha and Selcia’s partnership, [13C], [14C], [2H], [3H] and [15N]-labelled metabolites can be accessed to support regulatory, development or research projects in the pharma and crop protection industries. Hypha establishes optimized processes using unlabelled or stable labelled parent substrates, which can then be transferred to Selcia’s state-of-the-art radiochemistry labs for the production of radiolabelled metabolites.
We’ve observed an increase in requests for synthesis of N-glucuronides over the last couple of years. We speculate that this may be due to the increasing use of N-heterocyclic chemistry in the design of new small molecule drugs, and pan company strategies to reduce CYP metabolism. The situation is further complicated by the high interspecies variability in formation of some N-glucuronides, especially aliphatic tertiary amines and aromatic N-heterocycles. UGT1A4 and UGT2B10 are key enzymes responsible for N-glucuronidation reactions in humans, rates of which can be much higher than in other animals. To compound this, synthesis of N-glucuronides is not always straightforward, and can be further muddied by metabolite stability issues, complicating interpretation of data.


