Solving Challenging Drug Metabolites Project
Use of Hypha’s one-stop metabolite shop to deliver 100s of milligrams of 3 metabolites
Access to multiple metabolites needed to support clinical development is not always straightforward, and can sometimes mean that more than one technique needs to be applied to fulfil requirements.
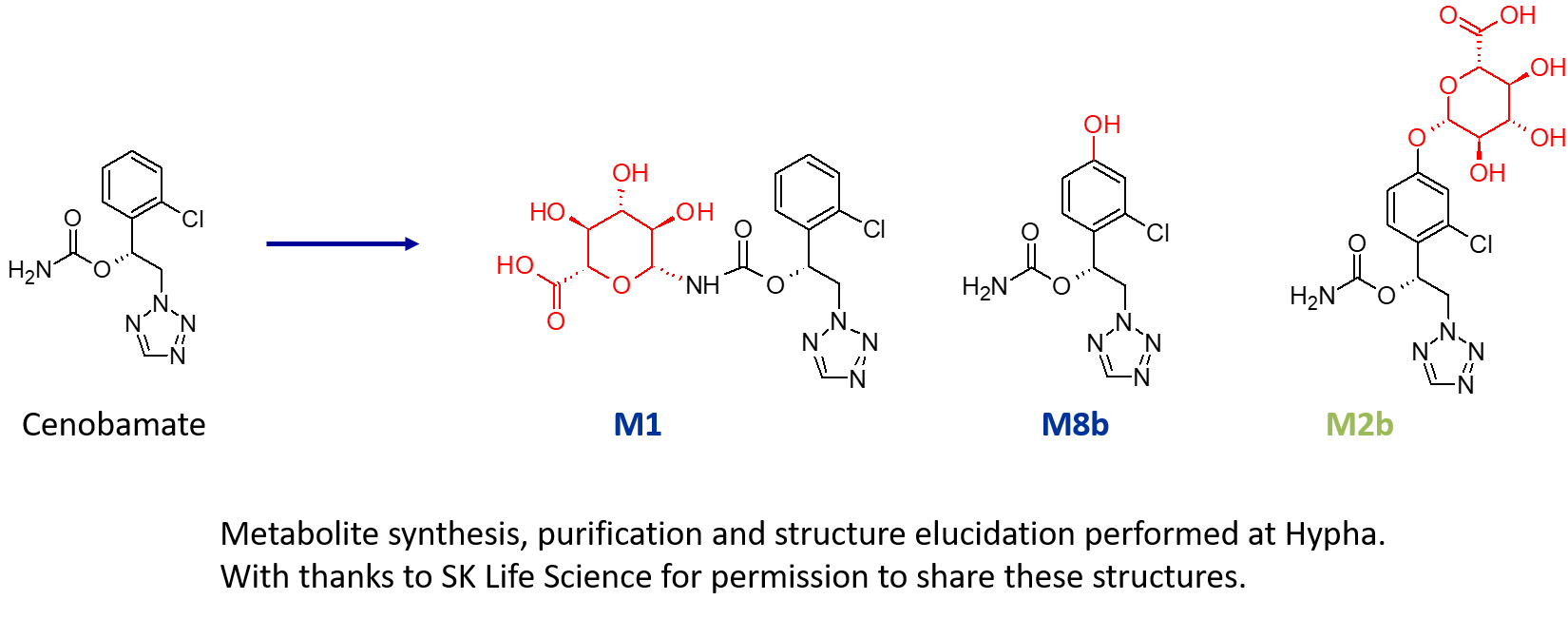
In one such project, a US pharma client required > 200 mg of three metabolites of a drug; an N-glucuronide (M1), an indirect O-glucuronide (M2b) and a hydroxylated metabolite (M8b). As part of this project, multiple components of Hypha’s one-stop metabolite shop were employed, including chemical synthesis, microbial biotransformation as well as purification and structure elucidation by NMR.
One-stop metabolite shop
Hypha’s one-stop metabolite shop allows synthesis and/or purification of all the main types of mammalian phase 1 and 2 metabolites.
Multiple techniques employed
Hypha are able to employ chemical synthesis, microbial biocatalysis, mammalian tissue fractions (multiple species S9s/ microsomes) and recombinant enzymes such as PolyCYPs.
It is our observation that access to N-glucuronides is an increasingly common need, as evident in this project where M1, a major N-glucuronide, was accessed using chemical synthesis. Key to successful synthesis were the mild deprotection conditions used in the late-stage chemical glucuronidation procedure, resulting in the purification of a gram of M1.
In addition to the N-glucuronide, an indirect O-glucuronide, M2b, and its aglycone, M8b, were also needed. To make M8b, the position of the hydroxyl group first had to be identified. To achieve this, Hypha chemists purified a small amount of M2b from human urine supplied by the client, and elucidated the structure of the conjugate using cryoprobe NMR spectroscopy. Then, knowing the position of hydroxylation from the structure of the phenolic glucuronide, 100s of mgs of M8b were synthesised. In order to access large amounts of M2b, a different approach was needed as this glucuronide was not amenable to chemical synthesis due to instability and formation of side products.
Instead, M2b was successfully made through microbial biotransformation of the aglycone M8b. Following a screen to determine the best microbial catalyst, 560 mg of M8b fed to actinomycete Species 45 was metabolised, from which 254 mg of M2b was purified.
Related resources
Formation and scale-up of human metabolites formed through mixed metabolic pathways is possible using Hypha’s microbial biocatalysis system. In vivo human metabolism of Incyte’s IND epacadostat (EPA) forms 3 major circulating metabolites, from both primary and secondary pathways. Glucuronidation of EPA forms M9, the dominant metabolic pathway, in conjunction with formation of an amidine M11 and an N-dealkylated metabolite, M12.
In Chapter 4 of the book on “Identification and quantification of drugs, metabolites, drug metabolizing enzymes and transporters”, Hypha authors summarise the different methods employed for producing metabolites of drugs, illustrated with representative examples from the literature and work undertaken at Hypha. The chapter also includes a discussion and examples of the use of NMR spectroscopy for structure elucidation of metabolites.
This poster illustrates the process undertaken at Hypha to produce a major human metabolite of ingenol disoxate for regulatory compliance.