One-Stop Metabolite Shop
Recombinant Enzymes
Recombinant Enzymes for Metabolite Synthesis
To access and identify phase 1 drug metabolites, Hypha have developed several recombinant enzymes. These include a set of proprietary diverse microbial CYPs called PolyCYPs®, as well as human recombinant aldehyde oxidase (AOX1) and all five human isoforms of flavin monooxygenase enzymes (FMOs). Each enzyme is expressed in E.coli and validated against a panel of relevant drugs and agrochemicals to demonstrate catalytic activity.
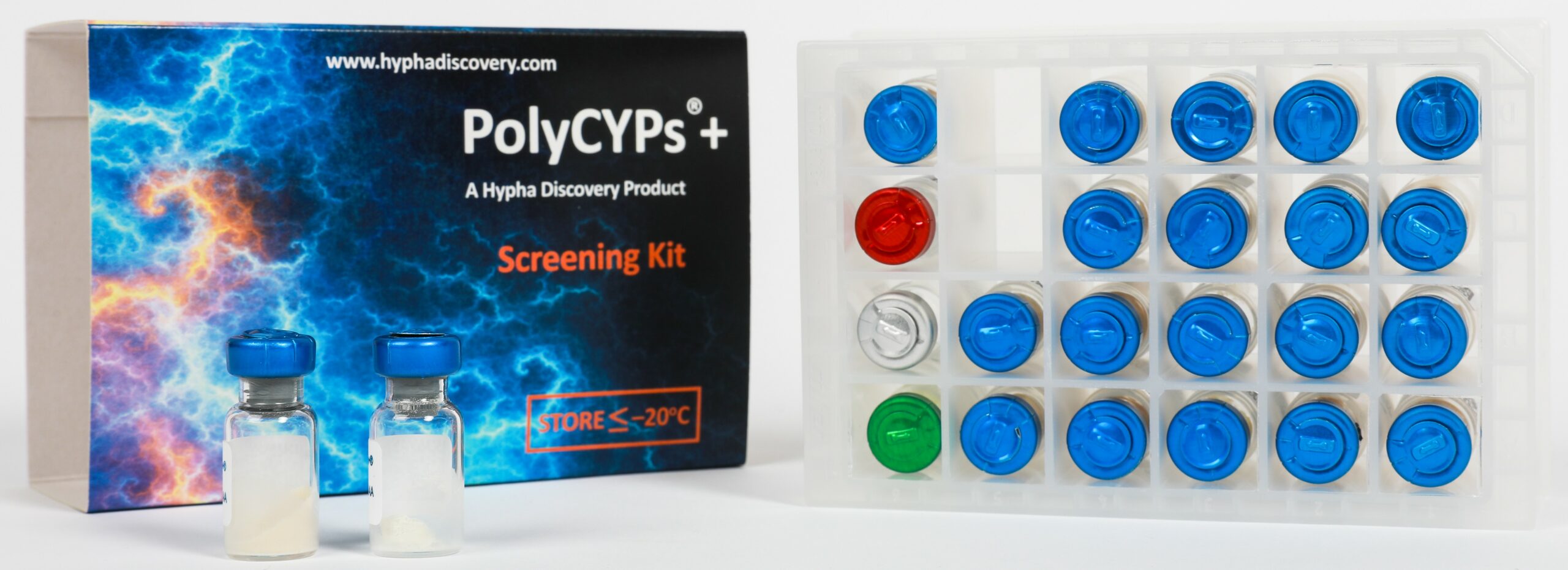
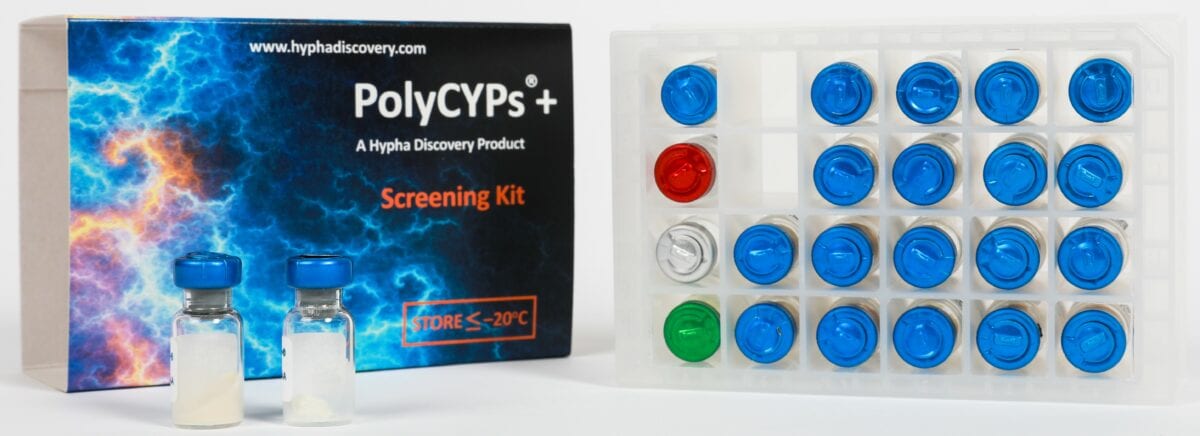
A set of 18 PolyCYPs enzymes, AOX1 and FMO3 are available in a screening kit form called PolyCYPs+,which clients can use in their own labs. The kit is fully supported through the availability of scale-up kits and the option of outsourcing the screening and preparation of larger amounts of metabolites to Hypha. We can also undertake larger scale-up projects, purification and structure elucidation of metabolites.
Our human recombinant aldehyde oxidase can also be purchased in a set of multiple vials for use in exploring biotransformation of drug candidates via this important route of metabolism. We have shown it’s effective in metabolising known AO drug substrates such as zoniporide, carbazeran and phthalazine.
For phase 2 conjugates such as glucuronides and glycosides, we have developed a set of recombinant UGTs cloned from some of the biotransforming bacteria in Hypha’s collection. These are a valuable set of tools we can use as part of Hypha’s one stop metabolite shop, alongside late-stage chemical synthesis and microbial biotransformation.
PolyCYPs+ Kits
PolyCYPs+ kits are effective for synthesis of phase 1 metabolites. The kits contain all co-factors needed to screen a drug substrate for metabolites, as well as a control compound and formulant for poorly water-soluble test compounds. All reagents are provided as lyophilised powders in kit form together with a 24-well reaction plate and air-permeable seal.
Once a target metabolite has been synthesised by one or more of the enzymes in the screening kit, a scale-up reaction with the best performing enzyme can be performed in order to access material for definitive identification of the metabolite and biological testing.
Higher amounts can be generated at Hypha, using either a bulk enzyme extract or through fermentation of a recombinant Streptomyces clone expressing the enzyme responsible for the biotransformation. We have been able to support production of gram amounts of metabolites for clients using this route.
About PolyCYPs
PolyCYPs are promiscuous class I and class III microbial cytochrome P450s (CYPs) mined from actinomycete bacteria in Hypha’s biotransformation panel, providing a wide diversity of CYPs that oxidise a variety of drug and agrochemical compounds. The enzymes are expressed in E.coli along with the redox partners ferredoxin and ferredoxin reductase. Although 18 PolyCYP isoforms have been selected to form a screening kit, over 100 isoforms form the PolyCYPs platform, access to which is possible through Hypha.
PolyCYPs mimic the action of human and other mammalian CYPs to produce human and other mammalian CYP-mediated metabolites. The use of multiple diverse PolyCYPs isoforms broadens coverage of reactions, which have been proven to metabolise some low turnover drugs and produce multi-step oxidised metabolites. In the example below, PolyCYP isoforms were able to produce the human metabolites of the slowly metabolised drug meloxicam M5 (66% conversion) and M4 (7.4% conversion).

Different PolyCYPs isoforms are capable of oxidising a broad range of substrate compounds, including aliphatic/benzylic, -CH, -CH2, -CH3, –tert-butyl, and isopropyl moieties, and aromatic systems for phenols and epoxides. De-alkylation of N– and O-alkyl moieties is also possible.
We are constantly improving PolyCYPs, including engineering new isoforms that widens the catalytic ability of the platform.
Case Studies
Rapid production of hydroxylated drug metabolites using PolyCYPs
A US client urgently needed at least 2 mg of a monohydroxylated metabolite (M4), originally observed in rat liver microsomes. Screening of the parent compound against 22 PolyCYPs enzymes and 23 microbes revealed the production of two main monohydroxylated products, one of which was M4. M4 was best produced by PolyCYPs 152 and 359, and by microbial biotransformation. The other monohydroxylated metabolite was produced by a different PolyCYPs isoform, (PolyCYP 350) and a wild-type bacterial strain.

Scale-up of PolyCYP 152 was selected as the quickest route for producing mg quantities of M4, prior to which a follow-up dose escalation study performed in 96-well plates showed that the substrate loading could be increased from 100 mg/L to 300 mg/L to provide higher volumetric yield of M4.
The scale-up reaction provided a higher conversion than the 11% conversion seen at the screening stage, increasing to 35% total conversion. A total of 20.1 mg of M4 was purified from the scale-up material using two rounds of HPLC purification. The metabolite was determined to be >97% pure by LC-UV-ELSD, and this was confirmed by 1H NMR. Lyophilised M4 was supplied to the client together with a Certificate of Analysis within 22 days from receipt of order, exemplifying the short timelines achievable using PolyCYPs to access CYP-derived metabolites.
Production of glucuronides by recombinant UGTs
Screening of a pharma client compound using multiple chemical and biotransformation methods revealed unique production of the two target O-glucuronides by a recombinant microbial UGT expressed in a Streptomyces strain. A 10L scale-up fermentation enabled purification of ~150 mg of the major glucuronide and ~20 mg of the minor glucuronide. Hypha elucidated the structure of both glucuronides using NMR spectroscopy and provided the pure material together with Certificates of Analysis to the client for bioanalysis studies.
In a project for an agrochemical client, four glucose conjugates were prepared. Two late-stage chemical synthesis methods provided three of the glucosidated metabolites. However one glucoside was only produced by biotransformation, with a different recombinant microbial GT giving 70% conversion of the parent compound. This illustrates the value of being able to employ both chemical and biological methods to access all of the required metabolites.
The glucosyltransferases have been shown to have a broad substrate range and are useful for N– and O-glucosylation.
Resources
Explore our library of resources comprising brochures, case studies, posters and publications about the work we do.
This poster illustrates the application of a new biocatalysis kit, PolyCYPs®, to enable scalable synthesis of CYP-derived metabolites of drugs. The PolyCYPs platform is comprised of a set of recombinant cytochrome P450 enzymes and redox partners cloned from some of the talented actinomycetes in Hypha’s biotransformation panel. Enzymes in the kit catalyze the oxidation of a wide variety of substrate types to generate multiple mammalian and microbial-derived CYP metabolites.
Access a brochure on Hypha’s PolyCYPs+ kits. The kits contain 20 enzymes effective for producing a wide range of phase 1 metabolites. As well as 18 PolyCYPs enzymes, the kit also contains human aldehyde oxidase (AOX1) and the main human hepatic flavin-containing monooxygenase (FMO3), with other human FMO isoforms also available from Hypha.
In this case study at least 2 mg of a monohydroxylated metabolite (M4), originally observed in rat liver microsomes, was required by a US pharma company. Using PolyCYPs, the target metabolite was supplied to the client together with a Certificate of Analysis within 22 days from receipt of order, exemplifying the short timelines achievable using PolyCYPs to access CYP-derived metabolites.
Other one-stop shop solutions for accessing and characterising metabolites
Hypha’s One-Stop Metabolite Shop enables synthesis, purification and characterization of all the main types of mammalian phase 1 and 2 metabolites.
Chemical Synthesis
Late-stage chemical glucuronidation
Mammalian biotransformation
Mammalian biotransformation
Microbial biotransformation
Proven panels of bacteria and fungi
Purification & structure elucidation
COAs, acquisition / interpretation of NMR data

Hypha Discovery was a huge help to our drug development timeline, when an ADME study revealed significant metabolites that were challenging to synthesize chemically. Hypha was able to rapidly reproduce the metabolites to confirm chemical structure, and then scale up to support nonclinical testing and bioanalytical method development, with far greater speed than chemical synthesis could achieve. The Hypha people were very pleasant to work with and the material they produced was of very high quality, which rounded out an overall great experience. I would recommend them without reservation.
Senior VP, Pharmaceutical Sciences
US Pharma company
Ready to begin? Our scientists are available to talk through your requirements
Hypha Discovery is a UK-based CRO supporting pharmaceutical and agrochemical companies worldwide through the production of metabolites and new derivatives of drugs and agrochemicals in discovery and development.
Resources
Cookie Policy | Privacy Policy | Supplier Code of Conduct Policy | Website Terms and Conditions
© Hypha Discovery 2021. All Rights Reserved.