Our Products
Recombinant FMOs
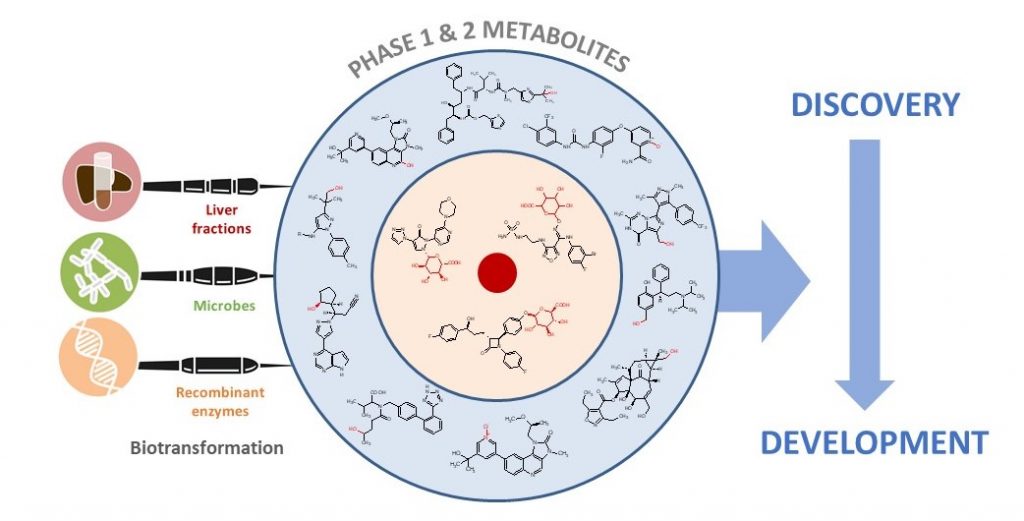
Accessing FMO-derived Metabolites
Metabolites derived from any flavin-containing monooxygenase (FMO) subtype can be produced as a service offering at Hypha. Recombinant enzymes of all five forms of human FMOs have been cloned into E.coli to provide a mechanism to access scalable quantities of FMO-derived metabolites.
FMO3, the most prominent form in human liver and associated with the bulk of hepatic FMO-mediated metabolism, is also available as part of Hypha’s PolyCYPs+ kit together with 18 PolyCYP isoforms and human AOX1 for accessing phase I oxidised metabolites. FMO3 catalyses N-oxygenation of primary, secondary and tertiary amines, as well as S-oxygenation of nucleophilic sulfur-containing compounds.
FMO metabolites may also be accessed through microbial biotransformation using some of the microbes in Hypha’s biotransformation panels
About FMOs
The involvement of FMOs in human metabolism of drugs is suggested to be underestimated (Phillips & Shepherd, 2016) and can offer an attractive route of elimination due to a lower tendency than CYPs to be inhibited or induced, resulting in a lower DDI potential. FMOs are a family of microsomal NADPH-dependent enzymes that catalyse the oxygenation of nucleophilic heteroatoms, particularly nitrogen, sulfur and phosphorus moieties. N-oxide and S-oxide formation represent the major FMO-mediated mechanisms, and particularly good substrates for FMOs include primary and secondary amines. Many drug substrates of FMOs contain tertiary amines (which are oxygenated to form the N-oxide), or sulfides, which are S-oxygenated to the sulfoxide. Most products of FMO metabolism are non-toxic however some of the N-hydroxylated products of primary and secondary amines can inhibit CYPs and may have toxic effects.
In humans, there are 5 functional FMOs which exist in different tissues in humans.
FMO1 has the broadest substrate range and is present in kidney, where it can contribute substantially to renal clearance of drugs, and the small intestine.
FMO2 is present mainly in the lung and also the kidney, however many humans do not produce a functional protein
FMO3 also has a broad substrate range and is the main hepatic FMO responsible for FMO metabolism in the liver.
FMO4 is present in the pancreas but is generally at low levels in tissues.
FMO5 is present in liver at levels similar to FMO3 but also present in stomach, pancreas, and importantly, the small intestine.
Notably FMOs are thermally labile, especially in the absence of NADPH.
Resources
Explore our library of resources comprising brochures, case studies, posters and publications about the work we do.
Formation of N-oxide metabolites is one of the major pathways for metabolism of tertiary nitrogen-containing drugs. Some N-oxide metabolites have similar or greater pharmacological activity to the parent drug and thus require exposure assessment. They can also be unstable and can revert to the parent drug.1 Conversion of an N-oxide metabolite back to the parent in vivo is a well- known phenomenon which may result in an altered tissue distribution of the metabolite and parent drug, such as that proposed for tamoxifen,2 or cause adverse reactions as reported for soratenib.3 In the lab, it is possible to reduce the potential for conversion of N-oxide metabolites to the parent through careful sample handling, as described for the clinically-significant metabolite loxapine N-oxide.4
In this paper, authors from Hypha and Incyte Corporation discuss the impact and application of biotransformation of drugs by mammalian systems, microorganisms, and recombinant enzymes, covering active and reactive metabolites, the impact of the gut microbiome on metabolism, and how insights gained from biotransformation studies can influence drug design.
In Chapter 4 of the book on “Identification and quantification of drugs, metabolites, drug metabolizing enzymes and transporters”, Hypha authors summarise the different methods employed for producing metabolites of drugs, illustrated with representative examples from the literature and work undertaken at Hypha. The chapter also includes a discussion and examples of the use of NMR spectroscopy for structure elucidation of metabolites.
See our other solutions for metabolite synthesis
Hypha’s One-Stop Metabolite Shop enables synthesis, purification and characterization of all the main types of mammalian phase 1 and 2 metabolites.
Chemical Synthesis
Late-stage chemical glucuronidation
Mammalian biotransformation
Panels of liver S9s / microsomes
Recombinant enzymes
PolyCYPs, hrCYPs, AOX, FMOs
Microbial biotransformation
Proven panels of bacteria and fungi
Purification & structure elucidation
COAs, acquisition / interpretation of NMR data

“We approached Hypha Discovery for the preparation of 10-50 mg quantities of two API metabolites that had proven difficult to synthesise chemically. The project was highly successful, combining beautifully executed multi-disciplinary science with clear and responsive communications; the collaboration was a genuine pleasure. I have no hesitation in recommending Hypha to others for metabolite generation and scale-up.”
Head of CMC
UK Pharma Company
Ready to begin? Our scientists are available to talk through your requirements
Hypha Discovery is a UK-based CRO supporting pharmaceutical and agrochemical companies worldwide through the production of metabolites and new derivatives of drugs and agrochemicals in discovery and development.
Resources
Cookie Policy | Privacy Policy | Website Terms and Conditions
© Hypha Discovery 2021. All Rights Reserved. Website by Fifteen.co.uk