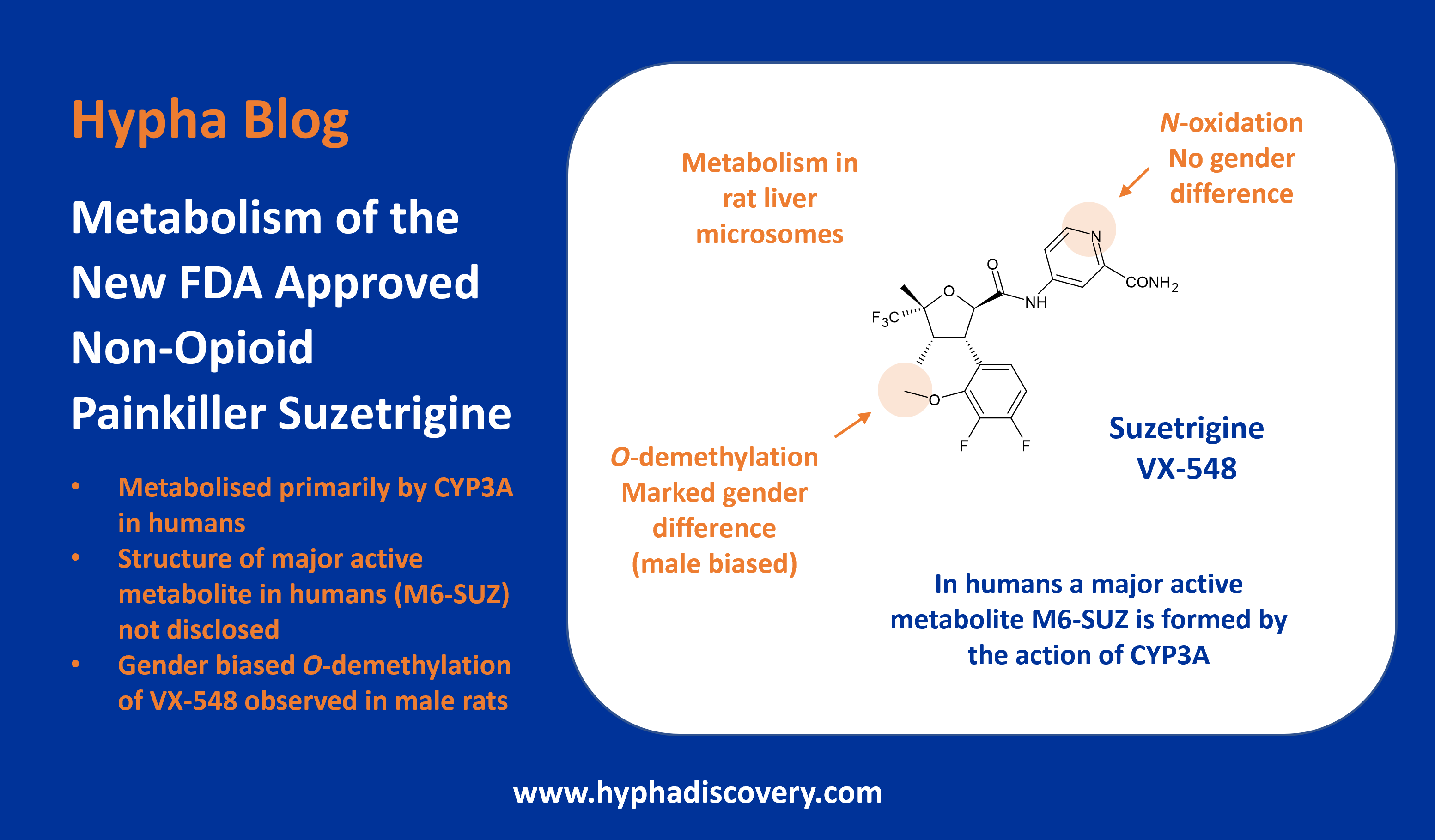
In vivo Active Aldehyde Oxidase Metabolite
Who knew that this active hydroxylated metabolite of a spiro-azetidino-piperidine drug, only discovered during clinical trials, would help unearth some previously unknown structure-activity relationships?
We’d seen the original paper on this in 2019 in which the importance of considering molybdenum containing oxidases during drug development was highlighted. Mentioned recently by Drug Hunter following a further paper digging into the pharmacology of PF-5190457, its largely aldehyde oxidase driven biotransformation reveals some intriguing differences about the metabolite.
It turns out that although the metabolite has lower binding affinity and potency at inhibiting GHSR1a-induced inositol phosphate accumulation vs the parent, it has increased potency at GHSR1a-induced β-arrestin recruitment.
The follow-up paper work characterising the pharmacology of the parent compound and the metabolite PF-6870961 revealed that PF-6870961 had no off-target interactions in a full HTS screen of binding and enzymatic targets. The authors demonstrated that whilst the metabolite had 25-fold lower affinity binding to GHSR1a than the parent, it was 4 to 7 fold more potent in recruitment of β-arrestin.
Interestingly the studies revealed some new insights into structure-activity relationships, which may impact future design of such GHSR1a inverse agonists. The metabolite is postulated to form hydrogen bonds in an amino acid region that confers biased inverse agonism of β-arrestin recruitment, possibly through disrupted flexibility in these regions.
The metabolite circulates at ~25% of the parent drug in humans and both significantly inhibit food uptake in rats at similar doses, demonstrating an in vivo effect of the metabolite.