What We Do
Custom Natural Product Synthesis
Custom Natural Product Synthesis
There is renewed interest in natural products as a source of chemical entities, either through discovery of new scaffolds or re-evaluating previously discovered bioactive chemicals. Microorganisms are renowned as a prolific source of natural products, many of which are medicinally important products. The enormous structural and chemical diversity of natural products is unmatched by any synthetic library of small molecules and natural products continue to inspire novel discoveries.
Hypha scientists are experts in the discovery, fermentation, purification and structure elucidation of microbial natural products and can support any aspects of projects in these areas.
The company has a collection of over 2000 higher fungal strains (basidiomycetes and ascomycetes) and 200 entomopathogenic fungi in addition to the bacterial and fungal strains used in its biotransformation panels. It has a high capacity for shaken flask fermentations and stirred tank bioreactors operating at scales up to 5 litres as well as a 50-litre airlift bioreactor. Hypha’s chemical scientists are highly experienced in natural product purification and the company’s chemistry laboratories are well-equipped for this purpose (for more information see the Purification page). We are also expert in structure elucidation by NMR, including new natural product scaffolds (see Structure Elucidation and NMR page).
In addition to the discovery of novel natural product structures, we are regularly asked to source known natural products not available to purchase off the shelf. For these projects, we refer back to the original literature relating to the compound(s) in question, and can often improve on the yields reported through application of our fermentation development and molecular biology expertise.
We work flexibly with clients to provide any aspect required, including the production and scale-up of stable and radiolabelled natural products. We are able to supply milligram to gram amounts of compounds and have access to multi-thousand litre scale reactors via an alliance with a fermentation provider.
What We Do
Examples of projects we have undertaken
Acquisition, maintenance and fermentation of microbial strains
Strain improvement and fermentation optimisation
Purification and structure elucidation of natural products derived from various sources (plants, microbes, marine organisms)
Purification of bioactive molecules from mixtures
Production of natural product scaffolds for semi-synthetic modification
Production and purification of natural product derived ADC payloads
Production of stable isotope-labelled natural products
Production of 3H or 14C radiolabelled metabolites and natural products, jointly with our partner Selcia
Hypha’s fermentation and Selcia’s radiochemistry expertise permits production of 14C-enriched semisynthetic and natural products by labelled precursor feeding. The first step involves sourcing and fermenting the microorganism to achieve acceptable yields of the product. Incorporation of an externally-fed 13C-labelled biosynthetic precursor to a fermentation is then undertaken, with confirmation of the location and extent of uptake of the 13C label. The process can then be replicated in Selcia’s facility to incorporate a synthesised 14C-labelled precursor into the final natural product.
Case studies
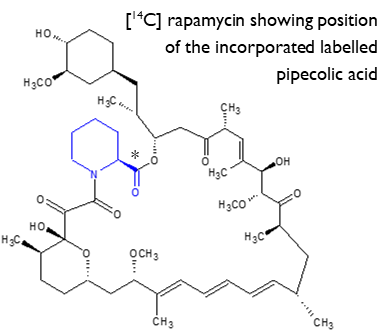
Production of a [14C]-labelled microbial natural product
Rapamycin is a microbial product of Streptomyces rapamycinicus and has potent immunosuppressive and anti-proliferative properties. Pilot work was successfully undertaken by Hypha to provide an optimised fermentation process for incorporation of 13C-labelled L-pipecolic acid into rapamycin. The process was then transferred to Selcia for production and purification of the 14C-labelled rapamycin from single labelled [14COOH] L-pipecolic acid, synthesised by Selcia using a short, efficient synthesis from 14C-labelled CO2. A total of 33mg of 14C-labelled rapamycin was purified at Selcia with a radiochemical and chemical purity of 94%, and a specific activity of 16.3 mCi/mmol.
Isolation of known and novel microbial natural products
Hypha was involved in a second EU FP7-funded project, NMTrypI, aimed at identifying chemical starting points for drugs to treat trypanosomatidic diseases. This paper describes the comprehensive technology platform involved and includes a description of an antitrypanosomal screening campaign using Hypha’s MycoDiverse library of fungal fermentation extracts, and the steps involved in screening hit dereplication and assay-guided purification.
Hypha has also been involved in an antileishmanial drug discovery collaboration with the University of Durham. This project used Hypha’s HDLSX library of fungal extracts derived from larger scale fungal fermentations. The paper describes the screening campaign and the assay-guided isolation and structure elucidation of a novel oxidised bisabolane sesquiterpene produced by a Marasmius sp. This compound demonstrated activity in an infected cell model and was shown to disrupt multiple processes using a metabolomic approach.
Why work with us
Our track record and experience in natural products chemistry across fermentation, purification and structure elucidation disciplines.
Our processes are scalable. Target molecules can be produced for further work from milligram amounts to assess bioactivity, up to gram scale and beyond for semi-synthetic modification.
Cost effective, flexible service. Clients may outsource entire programs or choose to work with us on a modular basis. We can also troubleshoot and rework existing processes and provide a full handover to clients.
Resources
Explore our library of resources comprising brochures, case studies, posters and publications about the work we do.
Leishmaniasis is a Neglected Tropical Disease caused by the insect-vector borne protozoan parasite, Leishmania species. Infection affects millions of the World’s poorest, however vaccines are absent and drug therapy limited. Recently, public-private partnerships have developed to identify new modes of controlling leishmaniasis. Most of these collaborative efforts have relied upon the small molecule synthetic compound libraries held by industry, but the number of New Chemical Entities (NCE) identified and entering development as antileishmanials has been very low. In light of this, here we describe a public-private effort to identify natural products with activity against Leishmania mexicana, a causative agent of cutaneous leishmanaisis (CL).
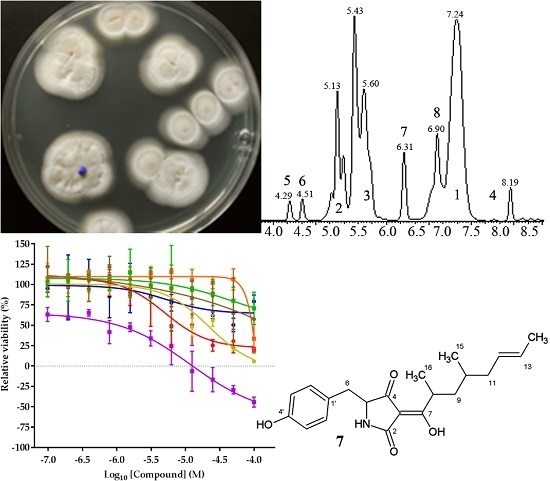
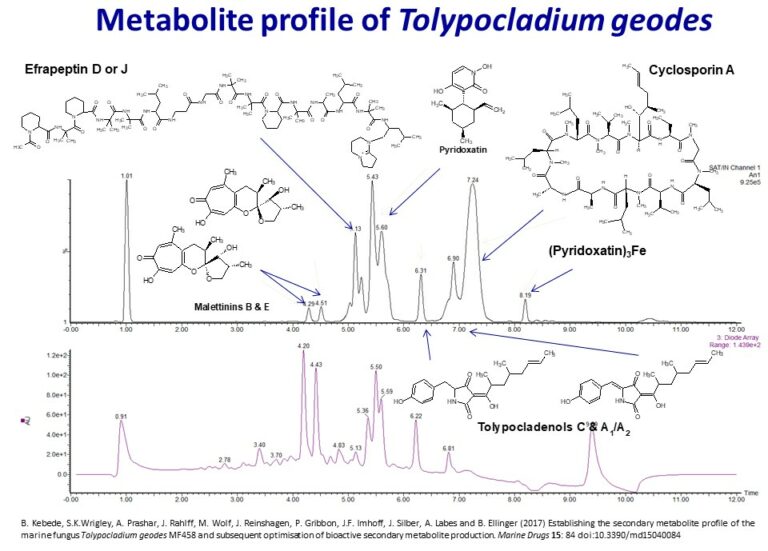
As part of an international research project, the marine fungal strain collection of the Helmholtz Centre for Ocean Research (GEOMAR) research centre was analysed for secondary metabolite profiles associated with anticancer activity. Strain MF458 was identified as Tolypocladium geodes, by internal transcribed spacer region (ITS) sequence similarity and its natural product production profile.
This study covers the isolation, testing, and identification of natural products with anticancer properties. Secondary metabolites were isolated from fungal strains originating from a variety of marine habitats. Strain culture protocols were optimized with respect to growth media composition and fermentation conditions. From these producers, isolated compounds were screened for their effect on the viability and proliferation of a subset of the NCI60 panel of cancer cell lines. Active compounds of interest were identified and selected for detailed assessments and structural elucidation using nuclear magnetic resonance.
Find out about our related services

We have used Hypha Discovery to provide samples of several pesticidal natural products by fermentation. We find them good people to work with. They provide a flexible and reliable service, meeting agreed deadlines, and so far have always provided us with the target compounds. We are pleased to recommend Hypha Discovery. Certainly we plan to continue to work with them.
Dianne Irwin, Principal Research Chemist
Syngenta, UK
Ready to begin? Our scientists are available to talk through your requirements
Hypha Discovery is a UK-based CRO supporting pharmaceutical and agrochemical companies worldwide through the production of metabolites and new derivatives of drugs and agrochemicals in discovery and development.
Resources
Cookie Policy | Privacy Policy | Website Terms and Conditions
© Hypha Discovery 2021. All Rights Reserved. Website by Fifteen.co.uk