What We Do
Late-stage Functionalisation
Late-Stage Functionalisation
Hypha scientists have developed a number of techniques to introduce oxygen and fluorine into drug molecules. We use either chemical or biocatalysis methods to oxidise positions selectively, optionally followed by fluorination to swap out hydroxyls for fluorine atoms. Chemical oxidation techniques in use at Hypha include use of multiple different reagents, electrochemistry and photochemistry.
Fluorine is unique in its ability to positively impact on several important properties, including pKa, biological activity, lipophilicity and, crucially for the medicinal chemist, metabolism. Use of deoxyfluorination at the lead optimisation stage provides a late-stage route to explore the possibility of blocking metabolic soft spots.
Biocatalytic late-stage oxidation
Hypha’s PolyCYPs enzymes permit in parallel late-stage functionalisation of lead compounds to multiple oxidised derivatives. PolyCYPs enzymes are cloned from some of Hypha’s microbes talented at oxidising drugs, and provide a mechanism to quickly screen lead compounds for hydroxylated and other oxidised derivatives.
PolyCYPs enzymes are available in kit form to allow reactions to be performed in client labs with the option of either a 20-enzyme screening kit (18 PolyCYPs, AOX and FMO3) or 8-enzyme (8 promiscuous PolyCYPs) diversification kit.
Hypha can also undertake screening of drug compounds using PolyCYPs through a service option called PolarExplorer. We operate this as a flexible end-to-end package which includes screening, scale-up, purification and optional structure elucidation of derivatives.
What We Do
Improving drug properties by late-stage hydroxylation
Biotransformation of drugs has led to intriguing findings. In particular, oxidation of drug leads by hydroxylation, subsequent oxidation to carboxyls, or dealkylation can lead to derivatives that have superior properties to the parent molecule. These derivatives may or may not be the major metabolites observed in the in vitro / in vivo system, as sometimes the minor metabolites or derivatives that might otherwise be overlooked, or not made in the specific in vitro system employed, may possess the improved properties.
Late-stage hydroxylation is a key tactic highlighted in a paper by Bostrom et al. in which examples of Hypha’s biotransformation technology were featured. The paper describes the potential benefits of hydroxylation, as part of a toolkit for medicinal chemists, which can result in improved activity, selectivity, solubility, and lipophilicity through replacing or mimicking water in binding site interactions.
Addition of a hydroxyl group can also influence pharmacokinetic (PK) properties as illustrated in the work by Stepan et al. where a PDE2 (phosphodiesterase 2) inhibitor lead compound at Pfizer suffered from exclusive clearance by CYP3A4. Through biotransformation profiling of the parent compound, several hydroxylated derivatives were produced, all of which maintained potency and also possessed higher metabolic stability. Whole cell biotransformation was used to produce hundreds of milligrams of one of these hydroxylated derivatives for ADME characterization using a streptomycete bacterium, a process subsequently able to be replaced by an 8-step chemical synthesis in order to provide multi-gram amounts for in vivo studies. The hydroxylated lead candidate was found to possess exquisite drug-like properties including a low oxidative turnover, increased renal excretion, and good selectivity against other phosphodiesterases.
Late-stage functionalisation methods available at Hypha
Chemical oxidation
Biocatalysis
Deoxyfluorination
Electrochemistry
Photochemistry
Case Studies
Late-stage hydroxylation of a drug lead to improve LLE
 Introduction of a hydroxyl group at the tert-butyl position of a Gilead kinase inhibitor using Hypha’s biocatalysis technology resulted in not only increased polarity but also an unanticipated 20-fold boost in potency such that the ligand-lipophilicity efficiency (LLE) was increased by 2.6 units to a more drug-like 6.0. This oxidised derivative was not a compound that would ordinarily have been synthesized, thus biotransformation provided an unexpected and valuable insight.
Introduction of a hydroxyl group at the tert-butyl position of a Gilead kinase inhibitor using Hypha’s biocatalysis technology resulted in not only increased polarity but also an unanticipated 20-fold boost in potency such that the ligand-lipophilicity efficiency (LLE) was increased by 2.6 units to a more drug-like 6.0. This oxidised derivative was not a compound that would ordinarily have been synthesized, thus biotransformation provided an unexpected and valuable insight.
“We piloted two compounds from two separate projects with Hypha, choosing examples that we knew to have moderate microsomal stability. We were very pleasantly surprised at the productive outcome where microbial incorporation of a hydroxyl group on a t-butyl substituent boosted the potency of a kinase inhibitor 20-fold, such that the LLE was increased by an extraordinary 2.6 units.”
Will Watkins
VP Medicinal Chemistry
Gilead Sciences, USA
Late-stage hydroxylation of a drug lead for exploration of SAR
In this case study, five active monohydroxylated derivatives of a big pharma client’s drug lead were produced using PolyCYPs, with a 56% conversion of the parent drug by the best PolyCYP enzyme, enabling access to polar chemical space in parallel as part of SAR studies.

Late-stage oxidation of risperidone
To complement our biocatalytic approach, we have also introduced extensive chemical oxidation conditions for late-stage functionalisation. In this case study with risperidone, application of a range of chemical reagents, electrochemistry and photochemistry resulted in the synthesis and identification of 14 oxidation products. This included the formation of the primary active metabolite of risperidone, and the main N-oxide degradation product.

Providing synthetic handles for late-stage fluorination
Fluorination is a well known strategy for improving metabolic stability of drugs. A late-stage hydroxylation-fluorination strategy can be used to test this.
Application of PolyCYPs to hydroxylate drugs in multiple positions, followed by a synthetic fluorination reaction enables exploration of the impact of fluorination in modulating CYP metabolism, particularly for those CYPs known to be implicated in drug-drug interactions or subject to genetic polymorphism.
In this case study, 4’-fluorocelecoxib was made via the intermediate 4′-hydroxycelecoxib. The fluorinated derivative is 4 fold more metabolically stable to CYP2C9 metabolism, but is metabolised faster in human liver microsomes, possibly due to metabolism by other CYP enzymes (Obach et al., 2016)

Read more about our deoxyfluorination capabilities here.
Reference
Obach et al., 2016. Biosynthesis of Fluorinated Analogs of Drugs Using Human Cytochrome P450 Enzymes Followed by Deoxyfluorination and Quantitative Nuclear Magnetic Resonance Spectroscopy to Improve Metabolic Stability. Drug Metabolism and Disposition May 2016, 44 (5) 634-646; DOI: https://doi.org/10.1124/dmd.116.069310
Resources
Explore our library of resources comprising brochures, case studies, posters and publications about the work we do.
Introducing oxygen into a drug candidate late in the optimisation process has several applications including exploration of SAR (structure-activity relationships) and the ability to access derivatives that may possess superior properties such as improved metabolic stability and LLE (ligand-lipophilicity efficiency). Biocatalysis can provide access to chemical space in a complementary manner to chemical synthesis and provide a “one-experiment” solution to accessing multiple derivatives in parallel. This poster illustrates the application of a new biocatalysis kit, PolyCYPs®, to enable parallel synthesis of hydroxylated derivatives of drugs.
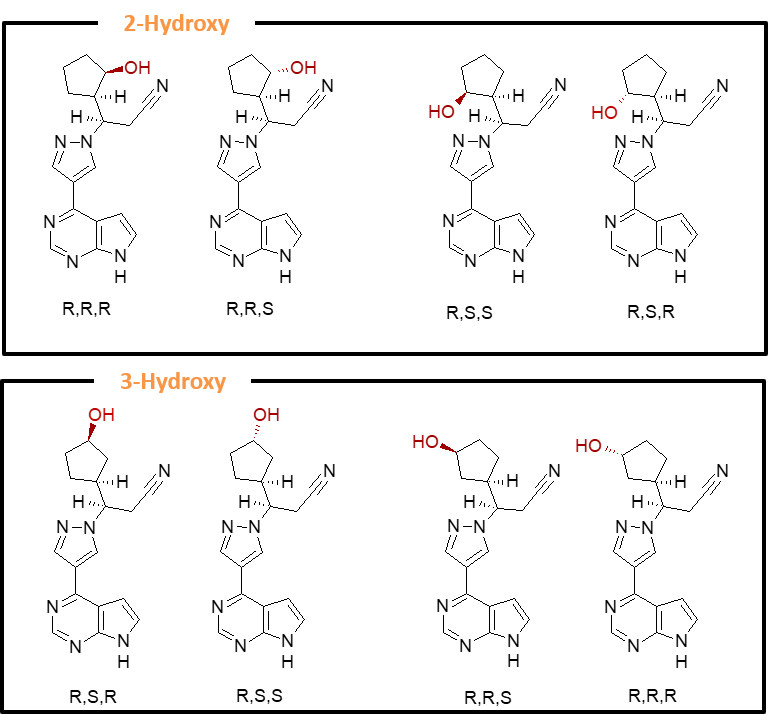
The first-in-class JAK inhibitor, ruxolitinib (trade names Jakafi® and Jakavi®), is primarily metabolized to a complex mixture of stereoisomeric cyclopentyl hydroxyl and keto metabolites. Application of Hypha’s microbial-based biocatalytic C-H bond activation to ruxolitinib resulted in the production of an array of hydroxylated and further oxidized keto metabolites, many of which corresponded to circulating human metabolites. All possible oxidized isomers of the aliphatic cyclopentyl moiety were derived from a variety of microbial species which were readily scaled up, enabling efficient production of stereoisomer metabolite standards for structural characterization and bioanalytical monitoring.
In this paper, authors from Hypha and Incyte Corporation discuss the impact and application of biotransformation of drugs by mammalian systems, microorganisms, and recombinant enzymes, covering active and reactive metabolites, the impact of the gut microbiome on metabolism, and how insights gained from biotransformation studies can influence drug design.
Find out about our related services

We would like to mention that it has been a pleasure to work with Hypha in this project. We are really satisfied with how the collaboration has progressed, reaching the defined goals and timelines in a very efficient manner. Access of hydroxylated compounds through PolyCYPs has saved our company time and resources. We are sure that we will have more opportunities to work together in the future.
Program Leader
European Pharma Company
Ready to begin? Our scientists are available to talk through your requirements
Hypha Discovery is a UK-based CRO supporting pharmaceutical and agrochemical companies worldwide through the production of metabolites and new derivatives of drugs and agrochemicals in discovery and development.
Resources
Cookie Policy | Privacy Policy | Supplier Code of Conduct Policy | Website Terms and Conditions
© Hypha Discovery 2021. All Rights Reserved.